|

What is Reverse Osmosis?
By David Walling
Osmosis is a natural process, known for over 200 years, on which reverse osmosis
systems are based. The walls of living cells are natural membranes. This means that the
membrane is selective, some materials can pass through, and others cannot. Most reverse
osmosis technology uses a process known as crossflow to allow the membrane to continually
clean itself. As some of the fluid passes through the membrane the rest continues
downstream, sweeping the rejected species away from the membrane. "Pure water"
is a relative term. Practically speaking, no water, naturally occurring or treated by man,
consists solely of the H2O molecule.
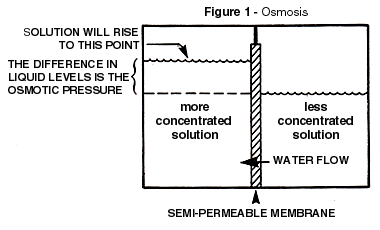
Figure 1 illustrates osmosis and the selectivity of the membrane. The semipermeable
nature of the membrane allows the water to pass much more readily than the dissolved
minerals. Since the water in the less concentrated solution seeks to dilute the more
concentrated solution, the water passage through the membrane generates a noticeable head
difference between the two solutions. This head difference is a measure of the
concentration difference of the two solutions and is referred to as the osmotic pressure
difference. This head pressure, converted to the familiar pressure units of pounds per
square inch (2.31 feet of water head equals 1 psi), allows the observation of a valuable
rule of thumb. That is, that each 100 mg/L total dissolved difference is equal to
approximately 1 psi osmotic pressure difference.
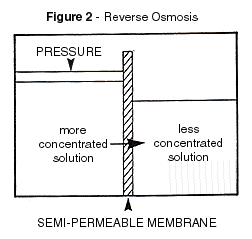 When pressure is applied to the concentrate side of the membrane
the difference of the osmotic pressure would be deducted from the net driving pressure.
The water passes through the membrane leaving most of the dissolved minerals behind. This
is where the term reverse osmosis is derived. The concentrate flow becomes a greater to a
lesser rather than a lesser to a greater through the use of applied pressure. When pressure is applied to the concentrate side of the membrane
the difference of the osmotic pressure would be deducted from the net driving pressure.
The water passes through the membrane leaving most of the dissolved minerals behind. This
is where the term reverse osmosis is derived. The concentrate flow becomes a greater to a
lesser rather than a lesser to a greater through the use of applied pressure.
Were the membrane to act as a perfect separator, the permeate would contain 0-mg/L
total dissolved solids, no matter what the concentration on the feed side of the system.
This is not the case, however. And, in fact, let us consider, for the sake of
illustration, 90% rejection to be an average operating condition. By considering the
mechanism of salt and water passage through the membrane, it will be clear why complete
salt elimination is not possible and how operating conditions can effect permeate quality
and quantity.
The membrane’s ability to hold back salts while allowing water to pass is based on
the fact that the salts are in solution as ions, that is, charged particles. The dissolved
salts are in solution as cations, with a positive charge, and as anions, with a negative
charge. A descriptive analogy of what is happening is to consider the membrane to be a
mirror. As the charged particles, ions, approach the membrane, they are repelled by a
reflection of their own charge. That is, similar charges repel, just as similar magnetic
poles repel each other. Therefore, the layer of water immediately adjacent to the membrane
is void of charged particle, and it is this water which will subsequently diffuse through
the pores and be delivered as permeate. Since the anions and cations are constantly moving
around in solution, sometimes they are near enough to each other to be attracted to one
another, thus canceling their individual charges. Without a net charge, these particles
are free to pass through the membrane.
Although Figure 2 was sufficient to illustrate the basic RO process, the feed and
concentrate ports added in Figure 3 are necessary to illustrate a continuously operating
RO system. It is the intent, in some applications of reverse osmosis that the concentrate
would be considered the product such as concentrating sugar out of a factories waste
stream.
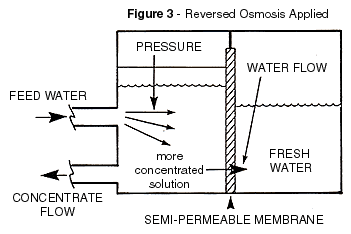
In order to keep the membrane from fouling it is important to continually flush the
brine side. As the water is squeezed through the membrane, leaving most of the salts
behind, the brine side solution becomes increasingly concentrated. Without the reject flow
to drain, the brine side mineral concentration would eventually exceed the solubility
limits of the salts present and they would precipitate, forming a scale on the membrane.
To avoid excessive brine side concentrations, the permeate volume recovered, in a
low-pressure system, is usually kept in the range of 1 to 5 percent of the feed stream
volume. For example, if for each five gallons of water fed to the membrane, one gallon of
permeate is recovered, the membrane is operating at 20% recovery. Operating RO systems at
a higher ratio of recovery will require more extensive pretreatment. Some reverse osmosis
systems would employ an automatic flush that will systematically allow more water to pass
across the concentrate side of the membrane. This feature is known as auto flush or manual
flush and will greatly increase the life span of RO membranes by flushing the build up of
biofilms and minerals out through the concentrate flow. This will however affect the
permeate flow rate during flush because it will allow more water to pass across the
concentrate side of the membrane decreasing the feed water pressure.
REVERSE OSMOSIS MEMBRANES
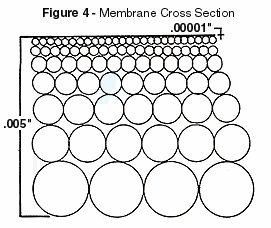
ConstructionThe semipermeable membrane used in most RO
systems are cast polymer films of asymmetric density. That is, they have a dense barrier
layer which is very thin, perhaps 10 millionths of an inch, supported on a more porous
substrate a few thousandths of an inch thick. The pores of the membranes that the
concentrate will diffuse through are 0.0001 microns in diameter. Just for comparison the
size of a human hair is close to 100 microns thick. Figure 4 illustrates the different
densities in the cross section of the membrane.
 . .
The most popular membrane configuration is the spiral wound, shown below in Figure 5.
Different configurations of membranes have been devised CTA and TFC membranes are the most
popular each offering certain advantages. CTA type membranes can be used in chlorinated
water using only a 5-micron sediment prefilter. Cellulose acetate membranes are limited to
a narrow pH range (2.5 to 8.5) and a lower maximum temperature, about 85F. These membranes
are biologically degradable. TFC type membranes offer a higher percentage rejection of
salts and a broader pH operating range from (2.5 to 11) and can tolerate a maximum
temperature of about 115F.
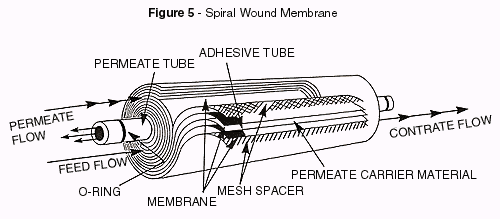
Sprial wound membranes, are assembled by folding a sheet of membrane over a perforated
tube. This tube is known as the permeate tube. A permeate carrier material also called
sail cloth is placed between the two halves of the membrane. The membrane is bonded to the
tube and glued together along the three open edges. Another spacer screen is laid on the
membrane and the whole sandwich is rolled tightly around the product tube and then bound
with tape to hold it together. Since the feed water must wind its way through the path
created by the spacer screen, dirt particles can be easily trapped, so 5 micron
prefiltration is generally recommended.
Reverse Osmosis Operation
The general operation of all RO modules is the same. The feed stream is supplied to the
membrane and split into the permeate which has diffused through the membrane, and the
concentrate which passes over the membrane, carrying away the minerals to waste. This waste flow should pass to drain via a
proper air gap so that contamination of the RO system does not occur if drain water backs
up in the drainpipe.
Low Pressure Systems
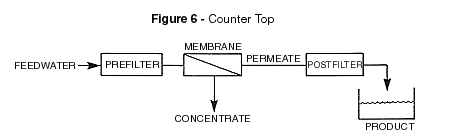 Low-pressure RO operation generally refers to feed pressures of
less than 100 PSI. This includes most of the equipment capable of being installed under
the kitchen sink and those referred to as counter top modules. Figures 6 and 7 define the
counter top and under the sink RO units. Low-pressure RO operation generally refers to feed pressures of
less than 100 PSI. This includes most of the equipment capable of being installed under
the kitchen sink and those referred to as counter top modules. Figures 6 and 7 define the
counter top and under the sink RO units.
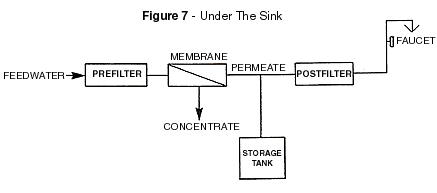
For counter top R O systems and most larger permanently installed units, the storage
tanks are maintained at atmospheric pressure - the majority of under-the-sink
installations utilize accumulator storage vessels where as water is added to the tank, the
air charge is compressed and thus the pressure in the tank rises. It is this elevated
pressure that is used to displace the drinking water from the storage tank to the faucet.
The accumulated pressure in the storage tank however, acts as backpressure on the
membrane, and as tank pressure increases, the differential pressure across the membrane
decreases. So as the tank pressure rises, the water production rate drops and yet the salt
passage continues unaffected. Thus the quality of the water being delivered drops
significantly if the differential pressure is allowed to become too low. Therefore, most
equipment included some provision for limiting the storage tank pressure to some value
less than line pressure. A ratio of two thirds is a commonly chosen limit as shown in the
configuration of Figure 8. New technology has been invented for the backpressure problem.
Known as a Permeate Pump this device uses the energy of the brine water running through
the RO system, to pump the permeate into your pressure tank. It does not require
electricity the permeate is propelled by using hydraulic energy that normally goes to the
drain unused. This isolates your tank from the membrane and lets your membrane perform
like in an atmospheric tank system without the backpressure.
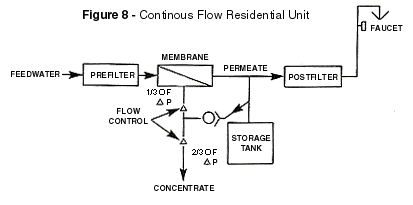
The flow control shown in Figure 8. is known as a Split Capillary. It is a very
inefficient design the average residential system will waste more than 35,000 gallons of
fresh tap water a year. This configuration should be converted to a shut off type system
shown in Figure 9. when found in residential applications. When the storage tank has been
filled to the point at which its pressure equals two thirds of line pressure, the permeate
is diverted to drain.
To conserve water consumption in reverse osmosis devices another type of control called
"shutdown" is employed in the design using a shutoff valves and is illustrated
in Figure 9.
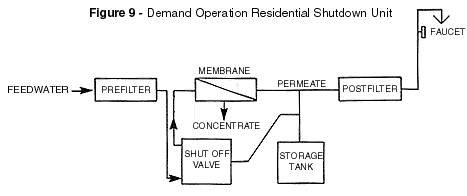
At the designed-in, present ration, the storage tank pressure will close the valve and
prevent further feed to the system. The valve will open again when sufficient pressure
reduction is sensed at the storage tank.
Whatever means is used to accomplish shut down, the end result is that the differential
pressure across the membrane is eliminated so that water production ceases. Unless
provision is made to eliminate the dissolved mineral concentration difference across the
membrane, salt passage will continue until the TDS level of permeate side of the membrane
is 50% of the original concentrate level. Example 500 PPM feed water with the pressure on
the membrane relaxed this would result in approximately 250 PPM on the concentrate side
and 250 PPM on the permeate side. The phenomenon is commonly referred to as a TDS creep
and is why most manufacturers of residential RO systems recommend draining the systems
storage tank at least once a month.
For more information,
please contact R/O CONN at (602) 432-5402 or fax (602) 942-1451. Or you can E-mail us at roinfo@roconn.com.
Back to Article & Press Release Index

Copyright © 1998 R/O CONN. All Rights Reserved
U.S. Patent # 5,660,720
|